There is little debate that well-fermented forages conserve dry matter, provide the opportunity for higher-forage diets and reduce purchased feed costs.
The main reason to inoculate forages is to provide insurance that forages will ferment properly.
Recently, I have calculated that with a corn price of $6.50 per bushel, an incremental 5 percent loss during fermentation equates to a cost in excess of $13 per ton of forage.
However, the strategy for inoculating is less clear. One challenge is that talking about the mode of action for inoculants requires a lot more understanding of chemistry in order to really understand what happens during fermentation.
With forage valued at $60 per ton, forage costs are exceeding $1,000 per cow per year. With this much money at stake, I feel that it is important for you to understand two different inoculant strategies and the chemistry behind both.
One strategy is to focus on immediate fermentation to conserve dry matter. Another strategy is to focus on aerobic stability during feeding. Aerobic stability is the time it takes feed to heat a couple of degrees after it has been exposed to air.
There is no doubt that heating during feeding is obvious to producers, but subtle dry matter losses caused by poor fermentation are usually more costly.
The chemistry of fermentation determines which of these two strategies are right for you. Unless you have just taken a university chemistry class, this next paragraph will be painful.
The two main products resulting from the fermentation of simple sugars are lactic acid and acetic acid. Lactic acid is basically a six-carbon sugar cut in half.
Lactic acid does not result in the loss of carbons, which conserve forage energy and is a strong acid. When you look across fermented forages, the final forage pH is closely related to lactic acid concentration.
On the other hand, when either simple sugars or lactic acid are fermented to acetic acid, which is a two-carbon molecule, the third carbon is lost in the form of carbon dioxide (CO2). As a result of this process, energy is lost and a water molecule is produced.
Sugar -> lactic acid + (acetic acid + carbon dioxide + water)
Consequently, this produces a 16.5 percent loss of carbon from the original sugar molecule. Production of water is also a reason that forages gain water (dry matter percentage decreases) during fermentation. Generally, acetic acid is considered a mold and yeast inhibitor.
However, there are two forms of acetic acid depending on the forage pH. The active form (AH) is able to inhibit molds and yeast while the other form (A-) cannot. For acetic acid, the ratio of active to inactive is 50 percent at pH 4.79. This is known as the pKa of the acid.
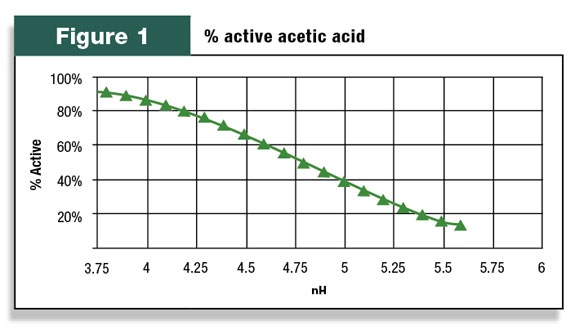
Figure 1 demonstrates how the percent active form of acetic acid changes with pH. At a pH of 5, only 40 percent of the acetic acid in forage is active against molds and yeasts.
Now back to the two main strategies for conserving forage dry matter.
The first strategy is to drop pH as fast as possible with “homofermentive” bacteria which produce only lactic acid.
By achieving a low terminal pH in four days, the highest amount of energy will be conserved during fermentation. To achieve this, a cascade of bacteria and an adequate supply of sugars are needed.
The foundation of this strategy is the bacteria called L. plantarum, which is known for its ability to grow at a low pH and to ensure a low final forage pH.
This strategy assumes that aerobic stability of the feed will be controlled through good bunk face management and achieving proper densities. What is interesting about this strategy is that the acetic acid level produced naturally by native bacteria is made more active due to the low pH of silage.
An alternative strategy is to focus principally on producing acetic acid for aerobic stability. This strategy is achieved using L. buchneri bacteria which convert lactic acid to acetic acid with a 33 percent carbon and energy loss.
For this strategy to be successful, forage pH must be maintained high enough for the L. buchneri bacteria to grow. L. buchneri is a slow-growing bacteria that is inhibited by low silage pH. As lactic acid is converted to acetic acid, forage pH will increase which renders acetic acid less active against molds and yeasts.
In nature, this process of producing acetic acid from lactic acid evolved to help decompose dead and decaying material. Acetic acid at high pH will not inhibit molds and yeasts.
An acetic acid content of 2 percent at a forage pH of 4.8 has the same mold and yeast inhibition as 2.6 percent at a forage pH of 5.
However, the energy loss of 2 percent acetic acid is less than that of 2.6 percent acetic acid due to conservation of carbon. When evaluating aerobic stability, it is important to put the acetic acid content in the context of pH.
In terms of specific strategies, using an L. buchneri bacteria with haylage is hard to defend. In this scenario, final pH will usually be above 5, which results in the majority of the acetic acid being in the inactive form.
Acetic acid is being produced with an energy loss without providing significant improvement in aerobic stability. Another strategy which is hard to defend is using an L. buchneri bacteria on wet corn silage. I caution against an L. buchneri on corn silage that has a dry matter content of 32 percent or less.
Research has shown that corn silage with a high level of water will have a natural tendency for high acetic acid content.
Consequently, promoting production of additional acetic acid from an L. buchneri bacteria is not advisable. On farm, I have also seen increased leachate from treating 32 percent DM corn silage with L. buchneri bacteria due to water that is formed when acetic acid is produced.
Use of inoculants as insurance can best be illustrated by understanding how they inhibit butyric acid. Butyric acid occurs principally in haylage when clostridia organisms are present and forage pH is high.
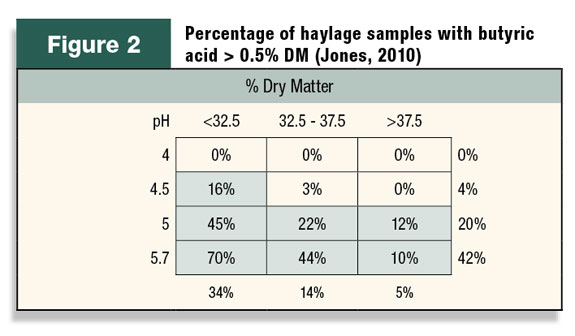
Table 2 suggests that haylage pH should always be under 5 to inhibit clostridia and, if the sample is wetter than 33 percent DM, the pH should be below 4.5 to ensure a level less than 0.5 percent butyric acid.
Butyric acid represents a significant energy loss but, more importantly, reduces intake and results in increased ketosis.
Forage values have increased along with rising commodity prices. Allowing forage fermentation to proceed without an inoculant that promotes a rapid pH drop represents a significant risk for dry matter loss as well as production of undesirable end products such as butyric acid.
Overall determinant of forage fermentation and feedout quality is pH. Corn silage should have a pH less than 4. Haylage should have a pH less than 5 unless it is wet, in which case, the pH should be below 4.5 to stop clostridia growth.
If you select an L. buchneri strategy, ensure that you are achieving acetic acid levels above 3 percent and that the final pH is low enough (less than 4.8) so that the majority of the acetic acid is in the active (AH) form.
It is important to recognize that dry matter losses during fermentation will increase as acetic acid (along with carbon dioxide and water) production increases.
However, an inoculation strategy which results in high acetic acid levels is important when air infiltrates forage for a significant amount of time before it is fed. Air allows yeast to propagate, which produces heat.
Inoculants reduce the risk of forage fermentation leading to undesirable results, but the two different inoculant strategies produce significantly different results.
A strategy of rapid pH drop after harvest minimizes fermentation losses, renders a higher percent of the acetic acid as active and relies on excellent bunk face management for keeping feed cool.
A strategy to promote acetic acid production results in a slower pH drop, more fermentation losses and the potential for less yeast growth during feedout (if pH is low enough). With the high costs of forages, it is important to select the strategy that is appropriate for your farm. FG
References omitted due to space but are available upon request. Click here to email an editor.
Click here to see a Counterpoint response.
PHOTO
Leachate can occur if proper conditions are not met and there is poor fermentation in the silage pile. Photo courtesy of Lawrence Jones.












