With the widespread drought this year, many forages contain higher levels of nitrate than normal. While testing and recommendations for toxicity levels are available, a little understanding of the biology of nitrate will help utilize nitrate-containing forage.
Nitrate (NO3-) is the primary nitrogen source for plant growth in most agricultural soils, though some nitrogen uptake in the form of ammonium (NH4+) occurs, especially in wetter soils.
To maintain ionic charge balance, every NO3− molecule taken into the root must be accompanied by either the uptake of a cation (like K+, Na+, Ca2+ and Mg2+) or the excretion of an anion (like a hydroxyl [OH−] or a bicarbonate [HCO3−] molecule).
In the plant, nitrate is reduced to nitrite (NO2−) in the above- ground plant cell solution. Nitrite is then reduced to ammonia in the chloroplasts and is incorporated into amino acids and proteins.
The steps of conversion in this process are as follows:
Nitrate (NO3) ——> Nitrite (NO2) ——> Ammonia (NH3) ——> Amino Acid ——> Protein
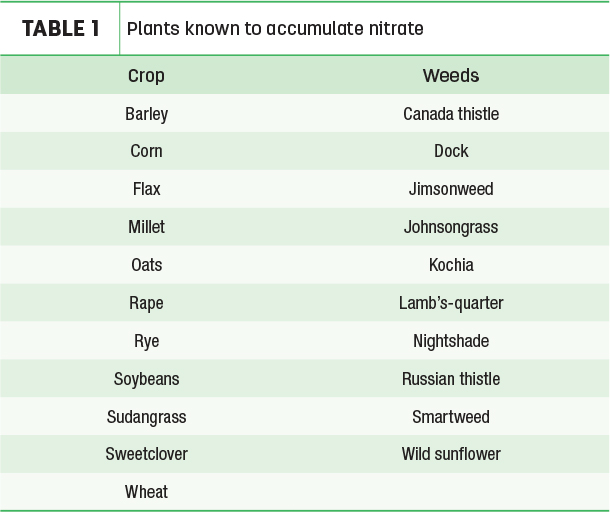
Conversion of nitrate to nitrite is an energy-requiring process. If the plant is stressed and less energy available, the rate of reduction is reduced, and nitrate will accumulate. Nitrite is approximately 10 times more toxic than nitrate. If fresh plant material or silage is being analyzed, one should ask for analysis of both nitrate and nitrite. Plant parts closest to the ground (stalks) contain the highest concentrations of nitrates; leaves contain less than stems; the flower and seed (grain) usually contain little or no nitrate. Additionally, some plants are known as “nitrate accumulators” – they do not take up nitrate more than other plants but rather convert it to nitrite more slowly than other plants so the nitrate accumulates.
Toxic nitrate levels are difficult to predict – both because nitrate concentrations can change rapidly in plants and the toxicity of nitrate varies greatly among livestock due to prior exposure, age, health status and diets. However, concern should certainly be raised when plant growth has been less than half of normal or nitrogen application more than twice recommended. Decreased light, cloudy weather and shading can also cause increased concentrations of nitrates in plants. Additionally, some herbicides, especially phenoxy herbicides such as 2,4-D, can increase plant nitrate.
Nitrate toxicosis can also result from accidental ingestion of fertilizer. Nitrate concentrations may be hazardous in ponds with extensive feedlot or fertilizer runoff.
Nitrate is converted to nitrite faster than nitrite is converted to ammonia in the rumen. When higher-than-normal amounts of nitrate are consumed, nitrite will be absorbed into the bloodstream and will convert hemoglobin to methemoglobin, which is unable to transport oxygen. Thus, when an animal dies from nitrate poisoning, it is due to a lack of oxygen.
At less than lethal levels, nitrate toxicity may result in poor performance, i.e., reduced weight gains or milk production. Clinical symptoms are rapid, weak heartbeat with decreased body temperature – muscular tremors, weakness and ataxia are early signs of toxicosis at methemoglobinemia levels of 30% to 40%. Brown, cyanotic mucous membranes develop rapidly as methemoglobinemia exceeds 50%. Anxiety and frequent urination are common.
Pregnant females that survive nitrate/nitrite poisoning may abort due to a lack of oxygen to the fetus; abortions usually occur 10 to 14 days after exposure to nitrates.
Milking cows and other animals receiving large amounts of grain are not as likely to have nitrate toxicity problems as dry cows, heifers and other animals because the milking cows are on a higher energy ration and because the high-nitrate feedstuff is likely to be a smaller proportion of the total diet.
Fermentation of feeds reduces the nitrate concentration; thus, with silage or fermented baleage, reduction of up to 50% nitrate is possible.
It should be noted that horses and other monogastric animals are not very susceptible to high nitrate levels as their bodies do not readily change nitrate to nitrite.
The good news is: Ruminants can adapt to higher nitrate concentrations if slowly introduced. Blending higher-nitrate feeds with lower-nitrate feeds is an alternative.
It’s important to also know the contribution of nitrates from your water source, especially in shallow or poorly cased wells in crop production areas. Both water and feed nitrate contributions should be considered when deciding whether to utilize a forage for your cattle.
Ruminant livestock can tolerate a wide range of nitrate, depending on several factors.
Factors making nitrate less toxic include:
- Healthy animals are less likely to be adversely affected than animals in poor health.
- The animal can become conditioned to eat larger amounts of feed with high nitrate content if the increase is gradual.
- High amounts of available carbohydrates (grain or highly digestible forage) allow the animal to consume more nitrate because carbohydrates enhance the conversion process from nitrate to microbial protein.
Factors making nitrate more toxic include:
- Rapid diet changes can trigger nitrate poisoning.
- Parasitism or other conditions causing anemia will increase susceptibility.
- Nitrate in more than one diet component (e.g., water and forage).
Management guidelines
- Dilute high-nitrate forages with other forages or feedstuffs low in nitrates. In some cases, this can reduce nitrate levels in the diet enough to make the forages safe to feed.
- Allow for frequent intake of small amounts of high-nitrate feed because that helps adjust livestock to high-nitrate feeds and increases the total amount of nitrate that livestock can consume daily without adverse effects (less than 9,000 parts per million nitrate).
- Give livestock access to fresh, nitrate-free water at all times.
- Supplement cattle grazing high-nitrate forages with other low-nitrate feedstuffs, such as low-nitrate forages, corn or sorghum stalks. The addition of high-energy feeds (feed grains or byproducts) stimulates the conversion of nitrate to non-toxic compounds and helps reduce the potential for toxicity.
- Be aware that cattle in poor health and condition, especially cattle suffering from respiratory disease, are more susceptible to nitrate poisoning.
- Consider harvesting and feeding high-nitrate forages as silage or baleage. Fermentation reduces nitrate levels; however, this does not guarantee that silage will contain “safe” levels of nitrate. Testing still is recommended.











