The International Plant Nutrition Institute (IPNI) ... in cooperation with university specialists ... has recently published a “how-to” workbook that presents commonly used mathematical concepts in agriculture.
It begins with simple arithmetic and advances all the way to complex modeling.
Most of us do not use sophisticated math on a regular basis, but a review of commonly performed calculations would be useful. We’ll start with some common calculations made when dealing with fertilizers.
Commercial fertilizers are required to show on their label the minimum percentage of nutrients the manufacturer guarantees to be present.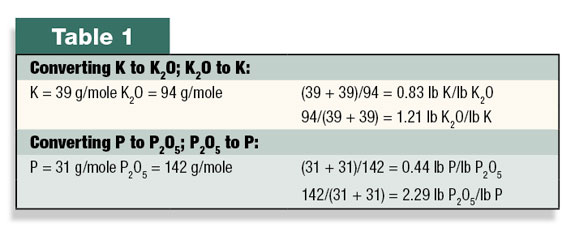
Fertilizer notation
The chemical analysis is composed of at least three numbers separated by dashes. The first number indicates the percentage of nitrogen (N), the second number indicates the percentage of phosphorus (P) as P2O5, and the third number shows the percentage of potassium (K) as K2O based on weight.
The nutrient content of the fertilizer is indicated by these three numbers, but the tradition of using the oxide form of P and K can be a bit confusing and is set in fertilizer law.
From the percent N value on the label, it is not obvious if the N is present as nitrate, ammonium or urea. Similarly, the P in most commercial fertilizers is chemically present as phosphate (PO4), but this number is mathematicaIly converted to P2O5 equivalents for display on the fertilizer label.
Potassium fertilizers are never present as K2O, but the K present in the fertilizer is mathematically converted to reflect this chemical equivalent. These conversions are done by comparing the ratios of molecular weight between them (see Table 1).
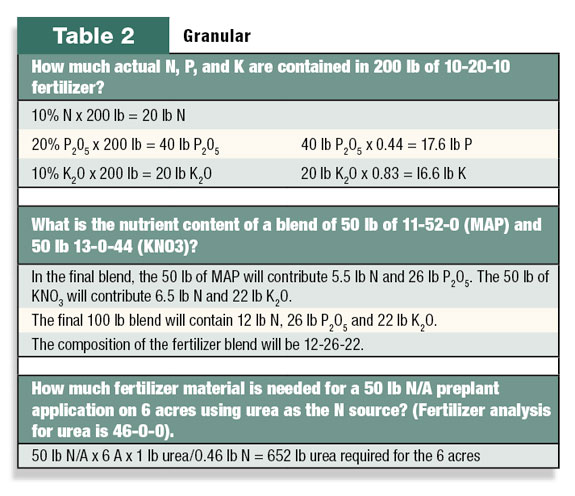
Some common questions regarding solid fertilizers can be answered with relatively simple math. See Table 2 for a few examples with granular fertilizer.
Liquid fertilizers
Liquid fertilizers are labeled based on the nutrient content in 100 pounds of material. These values are calculated the same as with dry fertilizers. However, fluid fertilizers are commonly applied based on their volume (gallons of material) instead of their weight.
Converting from a weight of liquid (pounds or tons) to a volume (gallons) requires knowledge of the liquid density. Density is defined as the mass of material divided by the volume.
For example, if a 5-gallon bucket of fluid fertilizer weighs 60 pounds, the density is 60 pounds/5 gallons = 12 pounds per gallon. It is important to precisely measure both the volume of the container and the weight of the added fertilizer (without including the weight of the container).
A complicating factor in determining fertilizer density is that most liquids become more dense in cold temperatures and less dense (expand) in warm temperatures. Luckily, these differences are relatively minor at the temperature range of most agronomic operations.
The density of a liquid is measured by floating a hydrometer in the material. This instrument provides a measurement of “specific gravity,” which is the ratio of the fertilizer density and water density. Once the temperature-corrected density of water is known, the density of the fluid fertilizer can be calculated.
There are also published tables available that show these density coefficients for various fluid fertilizers.
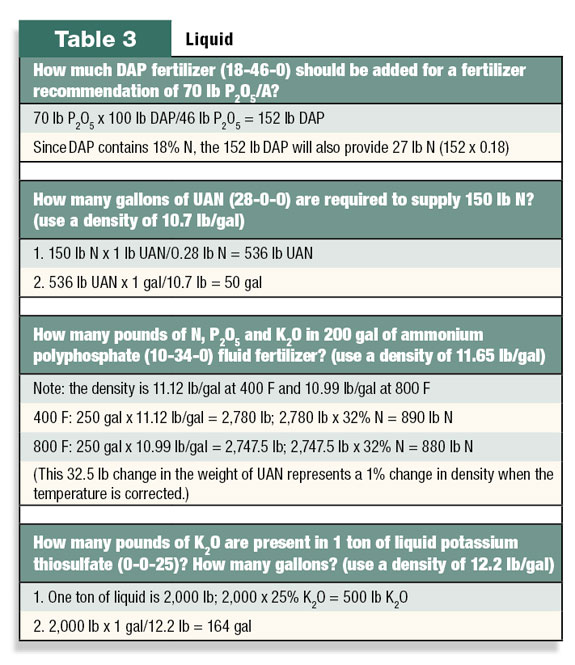
A few example calculations for liquid fertilizer are show in Table 3.
How much fertilizer should I apply?
To determine the amount of dry fertilizer to apply, the nutrient recommendation (usually given in pounds per acre (lbs/A)) and the nutrient content of the fertilizer must be known.
Calculating the quantity of fluid fertilizer to apply may require an additional step to convert the weight of the fertilizer to gallons of product delivered to the field.
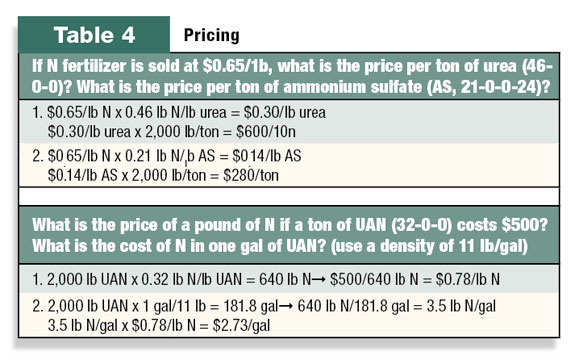
How much does it cost?
Fertilizer prices are usually provided in costs per ton (2,000 pounds) of material. Fertilizer prices are occasionally given in price per pound of N, P2O5, or K2O and not as a specific fertilizer product. The “per pound” terminology is sometimes referred to as a “unit.” Without close attention, this difference in pricing and terminology can be confusing.
Some sample calculations for cost are shown in Table 4. FG
—Excerpts from International Plant Nutrition Institute, January 2011
Robert Mikkelsen is the Western Director for International Plant Nutrition Institute and can be reached at rmikkelsen@ipni.net