By design, the scientific method has traditionally been sheltered from convenience. For scientific purists, a simple analysis is near sacrilege. After all, we’re supposed to suffer for our work, right? Science is supposed to be hard, so full of math and technical vocabulary that it brings a mere mortal to tears.
A recent insurgence of portable, affordable analytical instruments has challenged this paradigm. These devices are small, affordable and can be wielded by nearly anybody, with little to no scientific training required.
All of these “features” add up to a scenario that makes some shudder. What is science coming to? Have technological advancements in instrumentation truly been great enough that these measurements can leave the confines of the lab and maintain the same levels of accuracy and precision?
Looking specifically at the soil nitrate test, traditional laboratory-based methods have been in place for decades. Now there are in-field units that claim to remove the lab from the test. Can these be used as substitutes for laboratory analyses? To answer this question, let’s first look at how soil testing works.
The aim of any soil test is to determine the amount of a given nutrient the plant will find in the soil. These are known as “plant-available” or “exchangeable” nutrients. Plants, however, are not passive in their environment.
They are very active and can manipulate the chemistry of the soil environment immediately around the roots. As a result, there’s not really an exact definition of, or method to get to, a true “plant-available” value.
Keeping this in mind, a soil test consists of two basic parts: the extraction and the detection.
Extraction
The extraction is where the “plant-available” portion of the soil test comes into play. It is the act of displacing the nutrient of interest from the soil. In this case, it would be nitrate. Different researchers over time have developed different chemical solutions of varying strength and composition in an effort to best approximate what the plant sees in the soil. You may be familiar with some of the terms from reading your soil test results.
Phosphorus (P), for example, can be extracted with the Bray 1, Olsen or Mehlich 3 extractants. There are many more, but these are the three most common in the mid-western U.S. Each of these uses a slightly different mechanism to remove some (but not all) P from the soil.
The various extractants will sometimes remove similar amounts of P, and at other times remove very different amounts. How the different extractants compare among one another is not always straightforward. Soil properties, such as pH and clay mineralogy, can play confounding roles.
The traditional extractant for nitrate in the lab is two-molar potassium chloride (KCl). The extraction process is strictly controlled through an exact replication of the extracting solution, precise control over the amounts of soil and KCl, and a timed interaction between the two.
The laboratory also dries and grinds the soil to a uniform degree to try and eliminate as much background variation as possible.
In-field systems differ by using distilled water as the extractant and using a sample of field-moist soil. It’s possible that not all distilled water is created equal, given the many sources.
This is a potential error source that is not yet addressed. The amounts of soil and the soil/solution interaction timing, though, are well controlled in the in-field systems with which I have worked.
Detection
After extraction, there is the detection phase. Detection is the part of the soil test that quantifies the displaced nitrate. Detection methods and extraction methods can be mixed and matched, within reason, to create a complete soil test. In the case of the laboratory nitrate test, the extracted portion of the soil is filtered and the liquid is mixed with a chemical cocktail to create a bright pink color.
The intensity of this pink color is directly proportional to the amount of nitrate in the soil. In-field systems use an ion-selective electrode to measure the nitrate.
This is a handy method in that no additional chemicals are required to complete the analysis. It’s simply an ohmmeter that only reacts to the nitrate ion. The less electrical resistance, the more nitrate there is in the soil.
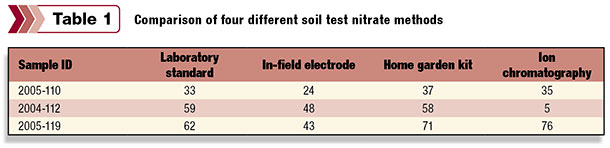
The combination of the extraction and the detection form the soil test method. In some cases, the soil test method employed can have a profound impact on the results. Table 1 shows how three different samples compare across four different soil test nitrate methods.
Which one is right?
As you can see, in some cases the results are very similar. In other cases, they vary significantly. But which one is right? The answer is surprisingly simple: They all are. This demonstrates the most important part of any soil test method – the interpretation. Researchers have spent years correlating soil test results to growing crops in an effort to understand exactly how test results can be translated into actionable management strategies on-farm.
The interpretation is where the new methods can create problems. Data mean nothing without the proper context. If the results of in-field analyzers are used as direct replacements for laboratory-based analysis, poor management decisions could result.
As an analogy, my vehicle has a digital speedometer. Last summer, I was driving through a residential neighborhood and, looking at the dash, realized I had sped up to over 40 mph. I panicked and hit the brakes. When I got back to 25, I felt I was just moving too slow. That was when I noticed that I had turned my display to kilometers per hour.
Once I turned it back to miles per hour, everything made sense again. My misunderstanding of the odometer led me to drive like a weirdo in somebody’s neighborhood. Proper frame of reference gives us an understanding of everything we do and helps us avoid mistakes.
When used properly, these instruments can provide a snapshot of what’s happening within the soil. There is a cautionary tale to tell here, though. It’s important to know that not all tests are created equal.
The analyzers described here have potential to lead the user astray if they are used as a one-to-one replacement for standard laboratory analysis. There is no substitute for a solid understanding of the science that goes into an analysis and the care that should be taken when using the resulting data.
What is the cost for the added convenience? When instruments are used properly, the cost includes the time and energy spent to fully understand the limitations and expectations of the methods.
When used incorrectly, the cost of such instrumentation will be sporadic poor management decisions, increased frustration and the heavy or light impact those can weigh on one’s livelihood. FG
PHOTO: Common market analyzers have potential to lead the user astray if they are used as a one-to-one replacement for standard laboratory analysis. There is no substitute for a solid understanding of the science that goes into an analysis and the care that should be taken when using the resulting data. Photo courtesy of Mikeal Dixon.












